Diagnosis of Leukemia – The Role of the Laboratory
Diagnosis of Leukemia – The Role of the Laboratory
Acute Leukemia
Rapid advances in understanding the molecular biology of acute leukemias are transforming the approach to diagnosis, prognostication and treatment of this diseases. This article will briefly review some of the basics of contemporary diagnosis of leukemia.
The 2008 WHO classification of Acute Leukemia is based on clinical features, morphology, immunophenotyping, cytogenetics and molecular genetics; it is a representation of distinct biologic and clinically significant disease subtypes.
Genetic changes in AML and ALL are probably the most important feature of prognostication.
The FAB classification which was based on morphology and cytochemical studies did not represent clinically significant subtypes.
2008 WHO Classification of Acute Leukemia
Acute myeloid leukemia with current genetic abnormalities
- AML with t(8;21)(q22;q22);(RUNX1;RUNX1T1)
- AML with inv (16)(p13.1q22)ort(16;16)( p13.1q22);(CBFB-MYH11)
- APL with t(15;17)(q22;q12);(PML-RARA)
- AML with t(9;11)(p22;q23);(MLLT3-MLL)
- AML with t(6;9)(p23;q34);(DEK-NUP214)
- AML with with niv(3)(q21q26.2);(RNP1-EVI1)
- AML with (megakaryoblastic)with t(1;22)(p13;q13); (RBM15-MLK1)
- Provisional entity: AML with mutated NPM1
- Provisional entity: AML with mutated CEBPA
- Acute myeloid leukemia with myelodysplasia-related changes
- Therapy related myeloid neoplasms
- Acute myeloid leukemia, not otherwise specific (NOS)
- AML with minimum differentiation
- AML without maturation
- AML with maturation
- Acute myelomonocytic leukemia
- Acute monoblastic/monocytic leukemia
- Acute erythroid leukemia
Pure erythroid leukemia
Erythroleukemia, erythroid/myeloid
- Myeloid Proliferations related to Down Syndrome
- Acute myeloid leukemia associated with Down Syndrome
- Acute Leukemia of ambiguous lineage
- Acute undifferentiated Leukemia
- Mixed Phenotype acute leukemia with t(9;22)(q34;q11.2); BCR-ABL1
- Mixed Phenotype acute leukemia with (v;11q23);MLL rearranged
- Mixed Phenotype acute leukemia, B/myeloid,NOS
- Mixed Phenotype acute leukemia T/ myeloid,NOS
- Mixed Phenotype acute leukemia, NOS rare types
- Natural killer (NK)-cell lymphoblastic leukemia/lymphoma
- Blymphoblastic leukemia/lymphoma,NOS
- Blymphoblastic leukemia/lymphoma with recurrent genetic abnormalities
- B Blymphoblastic leukemia/lymphoma with t(9;22)(q34;q11.2); BCR-ABL1
- B Blymphoblastic leukemia/lymphoma with t (v;11q23);MLL rearranged
- B Blymphoblastic leukemia/lymphoma with t(12;21)(p13;q22); TEL-AML1
- B Blymphoblastic leukemia/lymphoma with hyperdipoidy
- B Blymphoblastic leukemia/lymphoma with hypodipoidy
- B Blymphoblastic leukemia/lymphoma with t(5;14)(q31;q32); IL3-IGH
- B Blymphoblastic leukemia/lymphoma with t(1;19)(q23;p13.1): E2A-PBX1
Diagnostic Approach
- Clinical history & physical examination
- Complete blood cell count and Peripheral blood smear examination
- Bone marrow aspiration and trephine biopsy morphological examination
- Immunophenotyping of leukemia cells by flow of cytometry or immunohistochemistry
- Bone marrow cytogenetics studies
- Molecular studies in some cases
Clinical Features
Clinical findings play an important role in the 2008 WHO classification.
Patients with AML preceding history of MDS are diagnosed as AML with multilineage dysplasia.
If previously treated with chemotherapy, these cases are classified as therapy related AML; if a specific current abnormality is present these are diagnosed as AML with specific chromosomal abnormality eg. Therapy related AML eith t(9;11).
Patients with down syndrome are put in a separate category from the non-down syndrome cases.
Morphology
Laboratory evaluation of a suspected case of leukemia begins with peripheral blood examination; morphological evaluation of bone marrow aspirate and biopsy is recommended in all cases. Morphology remains the standard for enumeration of blasts which are expressed as a percentage of nucleated cells based on a 200 cell count in peripheral blood and a 500 cell count in marrow.
- In acute erythroleukemia the erythroid precursors are excluded from the blast count.
- In case with t(8;21),inv(16), t(16;16) or t(15;17) a diagnosis of AML is made even if the blast count is <20%
- If other genetic abnormalities are present and the blast count is <20% a diagnosis of MSD is made.
Morphological evaluation is essential for identification of multilineage dysplasia, for the identification of blasts with features of hypergrabnular or hypogranular acute promyelocytic leukemia and for detecting the presence of abnormal eosinophils in AML with inv (16) ort(16;16)
Immunophenotyping
Flow cytometric immunophenotyping is recommeded in all potential cases of acute leukemia. It is essential for the diagnosis of all cases of ALL, minimally differentiated AML and mixed phenotype acute leukemia. Identification of immunophenotyping patterns that correspond to specific disease groups is useful for guiding genetic studies.
Eg. Antigenic pattern MPO+/CD13+/CD33weak/HLA-DR+/CD34+CD19+characterizes AML with t(8;21)(q22;q22)
As aberrant expresson is frequesntly encountered, a battery of antibodies is required for definitive characterization of blast lineage.
Cytogenetics
Cytogenetics studies should be performed on all new cases of acute leukemia; recurrent cytogenetics abnormalities are found in approximately 40% of cases and defined risk groups in AML and B-ALL; favorable intermediate and adverse.
60% of cases are cytogenitacally normal (CN-AML) and are included in the intermediate group, however prognosis depends on specific molecular abnormalities.
In B-ALL to detect cryptic abnormalities such as t(12;21), molecular studies by FISH or PCR are indicated.
Cytogenetic indicators of prognosis are less clearly defined in T-cell ALL
Myelodysplasia-related cytogenetic abnormalities compromise complex karyotype, balance and unbalanced abnormalities, some of which occur most commonly in therapy related disease.
Molecular Studies
Cytogenetically normal AML (CN-AML) is a heterogamous group in which gene mutations are highly significant for prognostic stratification.
The class I mutations eg.FLT3 confer a proliferation and survival advantage; these occur late and are associated with disease progression.
Class II mutations CEBPA and NPM1 lead to impaired differentiation and survival advantage; occur early and are proposed to be initiating mutations. Class 1 and Class II mutations occur together, however class II mutations are mutually exclusive.
Class II mutations are associated with characteristic clinic-pathological features and possibly definite distinct biological entities.
- FLT3-ITDs occur in many subtypes of AML including 30% of CN-AML and are associated with a poor outcome.
- NPM1 mutations are the most common genetic abnormality in AML occurring in 30% of adult de novo AML and in 60% of CN-AML and are associated with a favourable prognosis in the absence of FLT3-ITD mutation, hence both mutations should be tested together.
- CEBPA is found in 5-9% of AML usually in M1 and M2 morphological subtypes and in 15% CN-AML; this mutation is considered to be an independent prognostic maker and is associated with a favourable prognosis.
Molecular studies by FISH and PCR are utilized for definitive diagnosis and to target therapy eg. APML with PML-RARA responds to specific treatment.
Current trials are exploring other molecular targets for therapy.
Monitoring levels of early treatment response and low level disease persistence provides an opportunity of early intervention.
It is expected that more targeted treatment will increase the demand for more sophisticated molecular testing
Role of clinical Laboratory in Acute Leukemia Diagnosis
- Peripheral blood examination
- Bone marrow; morphology
Flow cytometry immunophenotyping
Cytogenetic studies
FISH/PCR
Correlation with clinical features is essential for final conclusion
Chronic myeloid leukemia
Clinical features especially splenomegaly, indicate the need for further investigations for diagnosis of CML. Investigations comprise CBC & PBF examination, bone marrow aspirate and trephine biopsy morphology and trephine biopsy morphology and cytogenetic studies for Philadelphia chromosomes. FISH or molecular studies are indicated to detect the presence of the BCR-ABL fusion gene and for monitoring therapy.
Flow cytometric immunophenotyping is not necessary for the diagnosis of CML in chronix phase, however in blast crisis it can provide important information regarding the blast lineage and can also suggest disease acceleration.
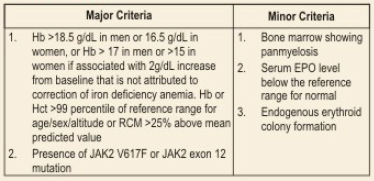
Polycythemia Vera
Diagnosis of PV requires both major criteria and one minor criterion or the presence of first major criterion and two minor criteria.
Chronic Lymphocytic Leukemia and other Lymphoid LKeukemias
Laboratory investigations recommended are:
- CBC and PBF examination
- Bone marrow aspirate and trephine biopsy morphology
- Immunophenotyping by flow cytometry
- Cytogenetic analysis
- Molecular studies by FISH or PCR
References:
- BetsBl, Hess JL Acute Myeloid Leukemia diagnosis in the 21st Century
Arch PatholLab Med 2010;134(10):1427-1432 - Arber DA, Algorithmic Approach to the classification of Leukemia; Unites States and Canadian Academy of Pathology(USCAP)
- Mahapatra M. RathiS POlycythemia Vera. Hematology Today 2010;113-117
- Kjeldberg RC Practical Diagnosis of Hematologic disorders 4thed.
….at National Reference Lab, Sector – 18, Rohini.
- Fully automated high throughput lab with rapid turnaround time (TAT)
- Cutting edge technology to deliver the most accurate test results from the widest test menu
- Dedicated central sample distribution facility for quick access and distribution of samples
- Conforms to strict international standards of quality and poised to handle the highest workload in Asia
- One stop solution for world-class diagnostics – pathology super specialized tests, radiology and cardiology
- An ergonomically designed and environment-friendly building