Enhanced Liver Fibrosis Test For Mapping Liver Functionality
Enhanced Liver Fibrosis: A Blood test aiding the assessment of liver fibrosis
Chronic Liver Disease (CLD) is a leading cause of death globally. Significant contributors include Viral Hepatitis B and C (HBV, HCV), Alcoholic Liver Disease (ALD) and Non-Alcoholic Fatty Liver Disease (NAFLD), although several other aetiologies exist and some of these causes may co-exist. Management of patients with CLD requires assessment and staging of fibrosis to identify those, who are at the most risk and in need of treatment or lifestyle modification. Suppression or reversal of fibrosis and possibly even early cirrhosis can restore liver functionality and minimize complications such as the development of Portal Hypertension or Hepatocellular Carcinoma. Biopsy has long been considered the gold standard for diagnosis, but in recent years, significant limitations have been acknowledged that compromise both sensitivity and diagnostic accuracy.
Non-invasive alternatives to biopsy have recently become available, including both imaging modalities and blood tests. Enhanced Liver Fibrosis (ELF) test is a novel blood test that measures levels of three direct markers [Hyaluronic Acid (HA), Amino-terminal Propeptide of Type III collagen (PIIINP) and Tissue Inhibitor of Matrix Metalloproteinase 1 (TIMP-1)] of fibrosis and utilizes an algorithm to generate a numeric score. Application of this score to patients with Chronic Liver Disease allows physicians to better assess fibrotic progression and can significantly reduce the number of patients requiring Biopsy. This article provides an overview of the current published evidence on the clinical utility of the ELF test.
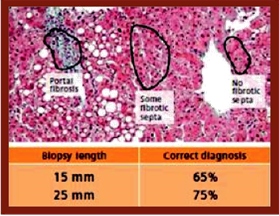
- Fibrosis is not always homogeneous within a Biopsy sample. Note the different conclusions that could be reached depending on the length of the Biopsy sample and the placement of the collection needle (Figure 1).
- Minor complications are relatively common with up to 30% of patients reporting post procedure pain. Severe bleeding extensive enough to require transfusion has been reported to occur in up to 0.04% of patients.
- Biopsy is inherently invasive and contraindicated in some (such as patients who are on anti-coagulation therapy and those with advanced cirrhosis).
- Patients are generally reluctant to undergo repeat biopsy, limiting its use in monitoring fibrotic changes and treatment efficacy.
Both imaging and serological methods have been developed as non-invasive and less invasive means of detecting and monitoring fibrosis. Both modalities have already been incorporated into HCV treatment guidelines as alternative methods to assess HCV-related liver fibrosis. Blood tests for fibrosis vary in design but usually utilize a panel of markers associated with liver damage, dysfunction or the fibrotic process itself. In contrast to Biopsy, which only evaluates approximately 0.002% of the total liver mass on average, blood tests (especially those utilizing markers directly associated with fibrosis versus just liver damage) offer the possibility of measuring the extent of total liver fibrosis and can supply clinically relevant information across the continuum of fibrogenic disease.
Direct markers of fibrosis measure biochemical markers of the fibrotic biochemistry itself. Fibrosis is a complex process involving both Fibrogenesis (scar tissue formation) and Fibrolysis (tissue repair, Figure 2).
The ELF markers and algorithm were originally investigated and validated in a large study of over 1,000 patients with multiple forms of Chronic Liver Disease including HCV, ALD and NAFLD, for the detection of fibrotic damage. Blood samples were obtained from patients within 6 months of Biopsy. Biopsy served as the reference standard and biochemical values used in the algorithm were correlated back to staging assigned by histopathology. To minimize the impact of subjective interpretation, all samples were assessed by a single expert pathologist and using the consensus of three expert observers for staging and grading. The final markers and algorithm (which are now referred to as the ELF score) showed clinically useful performance as assessed by AUC values for ALD, NAFLD and HCV (though performance varied somewhat, depending on the disease state). Subsequent work has established additional positive clinical performance for ELF, both in these common forms of liver disease and others, such as Primary Biliary Cirrhosis (PBC), Primary Sclerosing Cholangitis, Hepatitis B (HBV), and Autoimmune hepatitis. Adult and paediatric populations have been studied with ELF, with the test performing well in both. Both HA and PIIINP are involved in Fibrogenesis, TIMP-1 is actually an inhibitor in the pathway controlling fibrotic degradation. Thus, the three markers collectively capture both fibrogenic and fibrolytic associated activities.
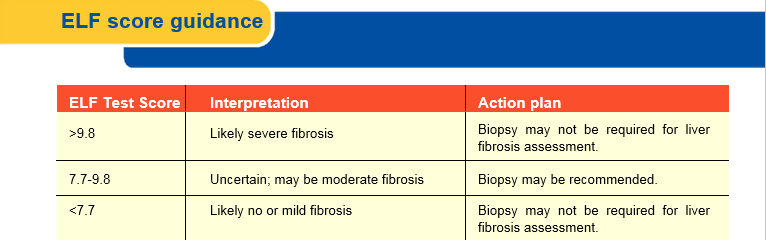
The ELF score provides a valuable enhancement over biopsy staging, in that, it is a continuous rather than categorical variable and is thus more sensitive to disease status and change. ELF score thresholds have been identified in populations of patients with known Chronic Liver Disease to identify these commonly used categories of fibrosis.
The low value of 7.7 was optimized for sensitivity, meaning any patient at or below that value has a low probability of any biopsy-proven significant fibrosis (none to mild). When used in a CLD population, a low ELF score can identify patients who are likely to avoid Biopsy safely (at least at the time of the ELF testing). Studies have suggested that as many as 43% of CLD patients could be safely ruled out for Biopsy using the ELF cut-off of 7.7 or less. Patients could then be monitored for any change, as fibrosis is a dynamic process and can change with time.
The high cut-point of 9.8 was optimized for specificity and is associated with biopsy-proven significant fibrosis. This means patients presenting with values greater than 9.8 are likely to have advanced fibrosis (to include cirrhosis) if they underwent Biopsy. Although a liver Biopsy is likely to be required for full assessment of the disease etiology and status, patients with high ELF scores could also likely avoid biopsy because the score indicates rule-in for significant fibrosis and instead be managed according to the specific cause of their CLD (e.g., antiviral therapy for HBV or HCV, alcohol abstinence for ALD and treatment of Non-Alcoholic Steatohepatitis [NASH]).
The percentage of patients presenting with advanced disease varies by population, so the percentage ruled in or out will vary with the testing cohort. However, as concluded by multiple investigations, it is abundantly clear that application of ELF to a CLD population could dramatically reduce the need for Biopsy. In a recent meta-analysis of ELF studies, the authors noted that overall, applying the ELF test to a diverse population of CLD patients showed considerable diagnostic performance for prediction of histological fibrosis stage. In addition, the authors found that ELF testing could reasonably allow 74% of patients avoid Biopsy. The impact of such a significant number of patients potentially avoiding biopsy cannot be understated, as it would reduce both cost and the morbidity associated with an invasive procedure. Even if a physician preferred to biopsy, availability of the ELF test could be useful for subsequent follow-up rather than attempting repeat biopsies.
- Liver disease severity should be assessed prior to therapy. Identifying patients with cirrhosis is of particular importance, as their prognosis, their likelihood of response and the duration of therapy are altered (Recommendation A1)
- Fibrosis stage can be assessed by non-invasive methods initially, with liver biopsy reserved for cases where there is uncertainty or potential additional aetiologies (Recommendation B1)
CLD patients with moderate ELF values would require additional assessment such as referral to Biopsy or secondary testing with an imaging modality. Studies have investigated possible synergy in using the ELF test in conjunction with an imaging modality and found this approach could further reduce the number of patients requiring Biopsy. Two approaches are possible: either utilize both technologies in the initial patient work-up or employ a sequential approach starting with ELF and then referring to imaging if ELF is insufficient for rule out or rule in.
The test has been well-validated in various CLD populations, as with many tests, optimal performance is influenced significantly by the prevalence of disease in the testing population. Specifically, if the ELF test is applied to populations in which the prevalence of fibrosis is low (such as to screen healthy individuals) the thresholds derived in patients with known CLD will result in the generation of many false positive test results.
Fibrosis is not unique to the liver, so extrahepatic sources of fibrosis arising from, for example, cardiac, pulmonary or kidney disease could theoretically contribute to an elevated score. For this reason, ELF (using the manufacturer’s recommended cut points) should not be used in a general population in an attempt to identify undiagnosed hepatic-specific fibrosis. Elevations of ELF also have been documented in other fibrotic disease states such as systemic sclerosis (and a clinical utility for ELF specific to this disease state has been studied).
An accurate characterization of liver fibrosis is critical in the optimal management of CLD. Distinguishing patients with significant fibrosis from those with benign disease has historically required an invasive biopsy, which can still fail to accurately assess damage. In addition, Biopsy is associated with pain, risk and substantial cost. The number of CLD patients is expected to grow substantially in the coming years, driven by anticipated increases in undiagnosed HCV infected patients presenting with advanced disease as well as the growing burden of NAFLD in many countries. For this reason, a direct and reproducible blood test for fibrosis offers great clinical appeal. The ELF test has been clinically validated in a range of Chronic Liver Disease states and the numeric.
ELF result allows the likelihood of fibrosis to be classified with good probability as none-to-mild, moderate or severe and helps target patients to the appropriate clinical pathway. Routine adoption of the ELF test into clinical practice presents an appealing alternative to both biopsy and imaging modalities that require significant capital investment, a trained clinician, and limited patient access. As with all other current alternatives to Biopsy, ELF can facilitate, but not completely replace the need for biopsy referral.
- The Performance of Enhanced Liver Fibrosis (ELF) Test for the Staging of Liver Fibrosis: A Meta-Analysis. PLOS ONE 2014; Volume 9 : Issue 4
- EASL Clinical Practice Guidelines: Management of hepatitis C virus infection. Journal of Hepatology, 2014; vol. 60: 392–420
- Rosenberg WM, Voelker M, Thiel R, et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127: 1704-13.