Everything You Need To Know About Breast Cancer Tests
- 8 Jun, 2017
- Written by Team Dr Lal PathLabs
Medically Approved by Dr. Seema
Table of Contents

Why Breast Cancer?
Breast Cancer is the most commonly diagnosed cancer in women, excluding non-melanoma skin cancer, and is also a common cause of cancer death, second only to lung cancer. In the past 15 years (2000 —2015) the proportion of the incidence of Breast cancer has increased from 20% to 25% in the top five cancers.
Diagnosis?
Diagnosis of Breast cancer is primarily made histologically, ancillary testing supports diagnosis, classification, prognosis, and prediction of response to therapy. Several well-established nonmolecular tests are part of the standard of care at first diagnosis of breast cancer as per the NCCN guidelines.
Some of the current molecular tests used in clinical practice today are mentioned in Table 1. (Hagemann et al. 2016)
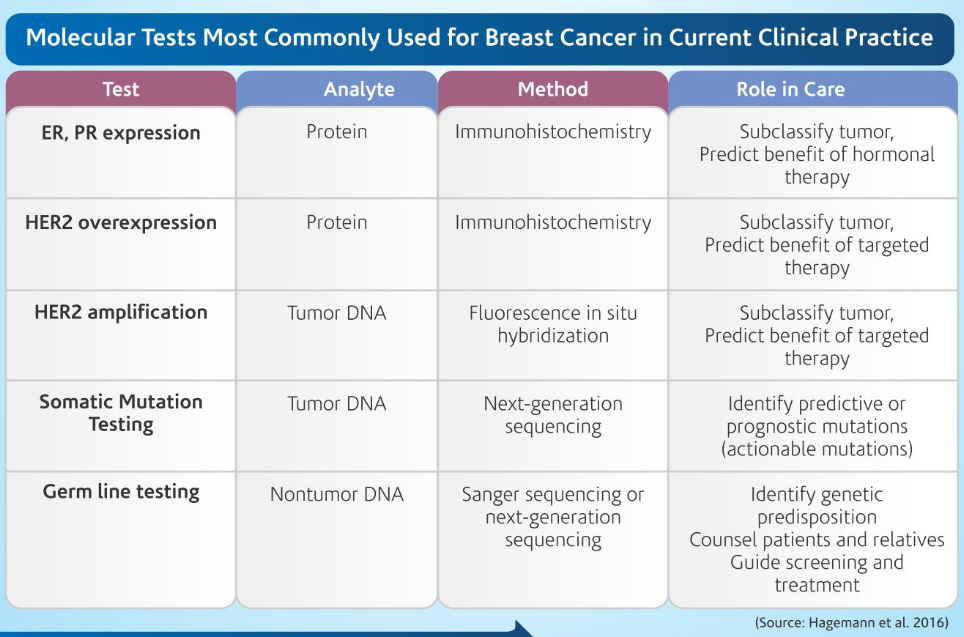
Why ER, PR and HER2 and Ki67?
Hormone receptor status predicts response to endocrine therapy. The National Comprehensive Cancer Network (NCCN) guidelines recommend that endocrine therapy be considered for any patient with Hormone receptor positive breast cancer test. For this reason, all new and recurrent breast cancers must be tested for ER and PR expression.
The HER2 gene is over expressed in 20% to 30% of breast cancer cases, because of copy-number variations (amplification). American Society of Clinical Oncology (ASCO) and the College of American Pathologists (CAP) have provided guidelines for HER2 Testing. Key aspects of the guideline include a recommendation that all primary, recurrent, and metastatic breast cancers be tested for HER2, either by immunohistochemistry (IHC; to detect overexpression) or fluorescence in situ hybridization (FISH; to detect amplification), using a validated test. The US Food and Drug Administration (FDA) approved assays are available for both of these methods of testing, and both are accepted as first-line testing. Both IHC and FISH have the potential to return a positive, negative, or equivocal result. Equivocal cases are recommended to undergo reflex testing by the other modality (eg, equivocal IHC cases are reflexed to FISH).
Though the NCCN Breast Cancer Guidelines do not currently recommend Ki-67 in routine clinical workup The Ki-67 proliferation index has been investigated as a breast cancer predictive and/or prognostic factor in various settings.
What is NGS and Does it help?
Tumor profiling is clinically available for detection of somatic mutations in breast cancer. These assays use NGS to detect potentially actionable variants in a set of cancer-related genes, and they may be of greatest use in triple-negative tumors.
Most familial breast cancer occurs in the setting of the hereditary breast-ovarian cancer syndrome (caused by mutation in BRCA1 or BRCA2), accounting for approximately 90% of kindreds for which a causative mutation is able to be identified.
Other inherited syndromes in which breast cancer is a major manifestation include Li-Fraumeni syndrome (TP53 mutation), Cowden syndrome (PTEN mutation), PeutzJeghers syndrome (serine/threonine kinase 11 [STK11] mutation), hereditary diffuse gastric cancer (cadherin 1ICDH11 mutation), and ataxia-telangiectasia (ATM serine/threonine kinase [ATM] mutation).
Assessment for hereditary cancer predisposition is part of the clinical management of breast cancer. Particular emphasis is placed on the evaluation of patients with certain risk factors: early age at onset of breast cancer,multiple primaries, male breast cancer, clustering of multiple syndromically related tumor types within the pedigree, high-risk ethnicity, and family members of individuals with known breast cancer susceptibility mutations.
References:
1. Hagemann, I. S. Molecular Testing in Breast Cancer: A Guide to Current Practices. Arch. Pathol. Lab. Med. 140, 815-824 (2016).
2. Wolff, A. C. et al. Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. JCO 31, 3997-4013 (2013).
3. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): breast cancer. Version 2.2017.National Comprehensive Cancer Network Website. https://www.nccn.org/professionals/physician gls/pdf/breast.pdf. Accessed April 28, 2017.
4. http://www.ey.com/Publication/vwLUAssets/EY-Call-for-action-expanding-cancer-care-in-ind ia/$ Fl LE/EY-Call-for-action-expa ndi ng-cancer-care-in-i nd ia.pdf













