First Private Lab in India to Introduce Electron Microscopy
Introduction
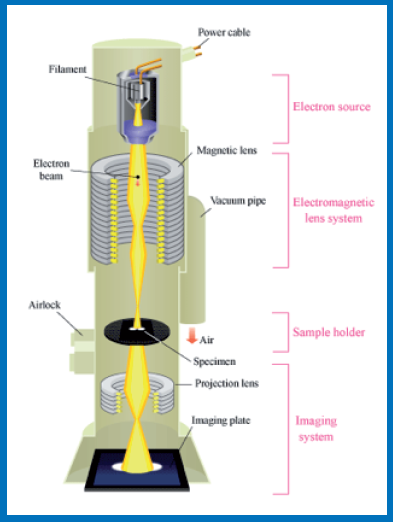
German physicist Ernst Ruska and the electrical engineer Max Knoll constructed the prototype electron microscope in 1931, capable of four-hundred power magnification. The first practical electron microscope was constructed in 1938 at the University of Toronto by Eli Franklin Burton and Siemens produced the first commercial Transmission Electron Microscope (TEM) in 1939. Transmission Electron Microscopy (TEM) is a technique in which a beam of electrons is transmitted through a very thin specimen, interacting with the specimen as it passes through and creating its image with the help of specially arranged electromagnetic lens system. A transmission electron microscope can achieve better than 50 pm resolution and magnifications of up to about 10,000,000x whereas most light microscopes are limited by diffraction to about 200 nm resolution and useful magnifications below 2000x. This enables a TEM user to examine fine detail which is thousands of times smaller than the smallest resolvable object in a light microscope.
Principle of TEM
An electron microscope uses a beam of accelerated electrons, usually generated by a Tungsten filament and travelling in vacuum as source of illumination. Because the wavelength of an electron can be up to 100,000 times shorter than that of visible light photons, the electron microscope has a higher resolving power than a light microscope and can reveal the structure of xtremely small objects.
Schematic diagram depicting parts of a Transmission Electron Microscope (TEM)
Basic procedures and sample processing
For a sample to be examined with TEM, it requires certain special procedures:
- Fixation: For most of the biological specimens, chemical fixation in 3% buffered glutaraldehyde stabilizes the specimen’s mobile macromolecular structure by chemical crosslinking of roteins and post fixation of lipids is done with osmium tetroxide.
- Dehydration: Replacement of water with organic solvents such as ethanol or acetone, followed by infiltration with embedding resins.
- Embedding: After dehydration tissue is passed through a ‘transition solvent’ such as propylene oxide or acetone and then infiltrated with epoxy resins such as Araldite, Epon etc.
- Semi-thin sections: After the resin has been polymerized (hardened), the sample is sectioned. First semi-thin sections are cut and stained with toluidine blue and suitable area is selected which is then subjected to ultrathin sectioning.
- Ultrathin sectioning: This produces thin slices of specimen, semi-transparent to electrons. These are cut on a special microtome called ultramicrotome using a diamond or disposable glass knife to produce ultra-thin sections about 60–90 nm in thickness.
- Staining: Ultrathin sections are stained for several minutes with an aqueous or alcoholic solution of uranyl acetate followed by aqueous lead citrate.
Applications of TEM
TEM finds application in diagnostic pathology, cancer research, virology, materials science as well as pollution, nanotechnology and semiconductor research. The high magnification of the electron microscope enables observations not possible by light microscopy and electron microscopy is considered to be an essential component of human diagnostic renal pathology, neuromuscular pathology and is a useful tool in difficult cases in oncosurgicalpathology. The use of immunoelectron microscopy enables ultrastructural localization of antigens of interest. Additionally, when samples for electron microscopy are inadequate, valuable diagnostic information can be obtained from ultrastructural investigations on reprocessed paraffin- embedded aterial.
Renal Pathology:
- In Renal Pathology, ultrastructural features enable a diagnosis to be made where the light microscopy is apparently normal. For example: minimal change disease, thin membrane disease, hereditary nephropathy/ Alport’s disease etc.
- Disease with glomerular deposits including fibrillary and immunotactoid glomerulonephritis, collagenofibrotic glomerulopathy, fibronectin glomerulopathy, dense deposit disease etc.
- Ultrastructural features provide information to confirm the diagnosis, as in immune complex mediated glomerulonephritis, renal amyloidosis, C3 glomerulopathy, diabetic nephropathy etc.
- Renal allograft Pathology: Early stages of chronic Antibody Mediated Rejection (AMR) have characteristic ultrastructural features, which develop much before LM features become evident and are vital clues in deciding therapeutic strategies in post-transplant period.
- TEM also enables structural and morphological diagnosis of viral infections. Negative staining techniques can be used to identify viral particles and other infectious agents.
Neuromuscular Pathology:
- In muscle fibres, the characteristic diagnostic features of several myopathies, glycogenic storage vacuoles, nemaline myopathy, actinopathies, and hyaline body myopathy etc. can be seen only with the use of TEM.
- Tumours of the CNS for which EM is useful include unusual or atypical variants of meningioma, ependymoma,schwannoma and oligodendroglioma-like tumours composed of small “clear” cells and small “blue cell” tumours of childhood.
- EM is also important in the evaluation of certain congenital, inherited and metabolic diseases including Neuronal Ceroid- Lipofuscinoses (NCL), CADASIL syndrome, mitochondrial encephalomyopathies and of certain toxic and drug-induced peripheral neuropathies.
Oncosurgical and General Surgical Pathology:
- In addition to the CNS tumours as outlined above, an important application of TEM is its utility in initiating a workup of an atypical tumour or metabolic condition, for which clinical and histological clues point in no obvious direction.
- Diagnosis of Primary ciliary dyskinesia and Kartagener’s syndrome where ultrastructural alterations in abnormal ciliary structures form the diagnostic clue.
Interesting cases
Case 1
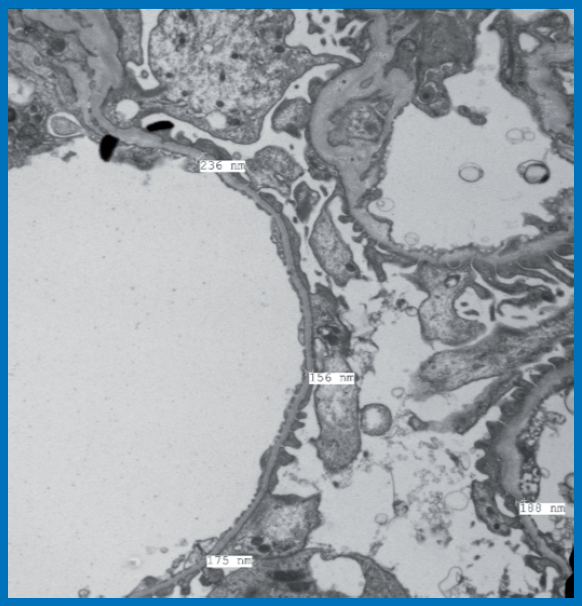
18 year old male, selected for Merchant Navy training, detected with microscopic hematuria on pre training routine medical examination. There was no family history of renal disease. Investigations revealed urine albumin-nil, RBC – 10-15/ hpf, serum Urea 15 mg%, Creatinine 0.7 mg%, no dyslipidaemia, viral markers negative, no urologic abnormalities, USG- normal sized kidneys with normal echogenicity and maintained corticomedullary differentiation. Renal biopsy was performed. Light microscopy showed morphologically unremarkable glomeruli, DIF studies were negative. Images form tissue processed for EM examination are depicted.
Ultrastructural examination: It showed largely maintained foot processes of visceral epithelial cells. No electron dense deposits or alterations of collagen structure in Glomerular Basement
Membrane (GBM) were noted. Analysis of GBM thickness at various levels showed majority of measurements below 200 nm. Mean GBM thickness was 201 ± 58.1 nm.
Diagnosis: Thin Basement Membrane Disease.
Discussion: The mean GBM thickness in normal Indian adults in few published studies varies form 321±29 nm and a cut off of 265 nm for diagnosis of thin basement membranes has been suggested. Present case showed majority of measurements below 200 nm, confirming the diagnosis of TBMD. Since this is considered a non-progressive abnormality, the subject was declared fit for admission to the course with advice of close medical follow up.
Case 2
11 year old male child presented with episodes of recurrent microscopic hematuria for last 6 months. Family history of renal disease on maternal side. Investigations revealed Urine albumin 1+, RBC 50-60/hpf, Creatinine of 1.3 mg%, viral markers negative, ANA negative, Complement levels – normal, USG- normal sized kidneys with mild increase in cortical echogenicity and maintained
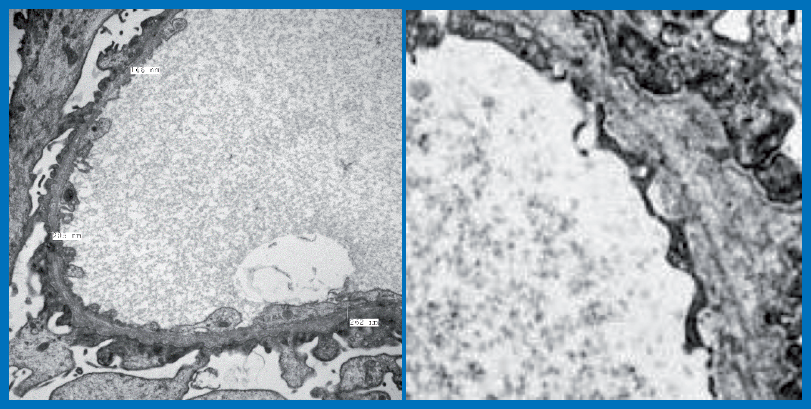
corticomedullary differentiation. No urologic, hearing or vision abnormalities were detected. Renal biopsy was performed. Light microscopy showed FSGS lesion in one of the sampled 8 glomeruli. DIF studies were negative. Images form tissue processed for EM examination are depicted.
TEM examination revealed patchy effacement of foot processes of visceral epithelial cells. No electron dense deposits were noted. GBM showed thick and thin segments and markedly disturbed
alterations of collagen with lamellations and “basket weave” pattern.
Diagnosis: Ultrastructural features are diagnostic of Alport’s disease (a genetic disease caused by mutations in type IV collagen genes (COL4A3, COL4A4 and COL4A5).
Transmission Electron Microscope facility at Dr. Lal PathLabs
Dr. Lal PathLabs is proud to be the first private laboratory in India to set up a Transmission Electron Microscope facility. We have a dedicated TEM lab housing state- of -the -art 120 kV JEOL Transmission Electron Microscope, located at the National Reference Laboratory (NRL), New Delhi. The facility incorporates a dedicated sample processing unit and is backed by a team of highly experienced & renowned pathologists and specially trained technical staff.
Sample collection for TEM
- Samples should be collected in special vials containing 3% buffered glutaraldehyde solution, which are available from NRL.
- Detailed clinical history in appropriate forms should accompany the specimen in all the cases along with contact number of the referring doctor.
- Turn Around Time (TAT): Seven days from the receipt of sample in the department.
Test range available
- J154: Transmission Electron Microscopy
- Z830: Kidney biopsy panel 3, which includes light microscopy with special stains, DIF examination and TEM studies
* Conditions Apply