HIV Virus Load by Real-Time PCR (RT PCR) Assay
HIV Virus Load by Real-Time PCR (RT PCR) Assay
Background: Progressive HIV infection causes an increase in plasma HIV-RNA levels accompanied by a corresponding decline in CD4+ T cell count. Absolute CD4+ T cell count has been used as a criterion to initiate antiretroviral therapy (ART). However, CD4+ T cell count alone is inadequate predictor for initiating antiretroviral treatment since T cell is subjected to enormous variations due to age, sex, various socio-economic and biological factors. On the other hand plasma HIV-RNA levels, depicting the actively replicating circulating virus, contribute more important information particularly in early HIV infection, guiding individual treatment decisions, evaluating prognosis, determining the rates of mother to child transmission and assessing the growing trend of antiretroviral drug resistance. Recent studies have shown that changes in plasma HIV virus load precede changes in CD4+ T cell counts by 8 to 12 weeks. Thus, it is considered to be the best predictor of the disease progression as well as effectiveness of ART.
Development of new molecular techniques designed to detect circulating virion- associated HIV RNA in plasma has created an opportunity to study viral dynamics and HIV pathogenesis in substantial detail. Of the three quantitation assays namely Real-time Reverse-transcriptase Polymerase Chain Reaction (Real-time RT-PCR) assay, Branched DNA assay and Nucleic Acid Sequence-based amplification assay Real Time RT-PCR being a kinetic assay provides better quantification of the initial copy numbers than end-point measurements employed by the other assays. The dynamic range of Real-time RT-PCR assay makes it particularly useful for quantifying full range of virus load observed in treated and untreated patients with HIV infection.
Real-time PCR is a powerful advancement of the basic PCR technique. The older PCR technique is essentially qualitative in which the end point amplification products are visualized on gel-electrophoresis in a post – PCR processing step. This step is a major source of cross – contamination and false positive results. Moreover, conventional or basic PCR is only able to detect the 10 fold changes in the endpoint amplified product, thus increasing the chances of a false positive result. It also has a low sensitivity of detecting only 500 copies of the target nucleic acid in the sample. On the other hand, Real-Time PCR is essentially quantitative method, which offers dynamic range of detection of the target nucleic acid during exponential phase of amplification. Through the use of appropriate fluorescent detection strategies in conjunction with proper instrumentation, all important starting amount of nucleic acid in the reaction can be accurately quantitated. Quantitation is achieved by measuring an increase in fluorescence during the exponential phase of PCR. It is able to detect even two fold changes in the concentration of the product. The test is performed in a single tube and no post-PCR processing step is required to detect the end-point product, thus reducing the possibility of false positive results to almost negligible, thus enhancing the sensitivity of the assay multi fold. Taqman version of the assay is highly specific with a sensitivity of less than 50 copies of the target nucleic acid in the sample. Applications of Real-Time PCR include measurements of viral load, gene expression studies, clinical diagnostics, and pathogen detection.
When feasible, the same assay should be used for serial plasma viral load testing in the individual patient
Plasma viral load serves as an invaluable marker for monitoring the progression of HIV infection and the efficacy of antiretroviral therapy in HIV infected patients. Despite obvious importance of HIV RNA levels in disease prognosis, minimum information is available on viral set points from developing countries like India. A major hurdle faced in the developing countries is the non-availability of cost-effective assay for the measurement of plasma HIV RNA levels. Moreover, the available costly commercial assays for viral load testing that are used in clinical settings were initially designed primarily to assess HIV-1 subtype B infection. Although some of these assays have been improved in terms of sensitivity and specificity for the detection of non-B subtypes of HIV-1, there are reports of discordant viral load results with non-B subtypes particularly subtype C. Measurement of HIV reverse transcriptase enzyme activity or p24 antigen levels by certain commercial kits provides alternative low cost means to quantitate viral loads. However, the sensitivity of such assays remains a major concern for the routine use of these kits for patient monitoring. Also, it is recommended that the same assay be used for periodic monitoring at multiple time points for individual patient due to the inter-assay variability in viral load testing. Therefore, a simpler, cost-effective and sensitive assay is urgently needed for longitudinal HIV disease monitoring in HIV- infected individuals.
Since in India subtype C is the predominant circulating HIV-1 clade accounting for more than 90% infections, we at Dr. Lal Pathlabs Pvt. Ltd. conceptualised development of a sensitive, specific, reproducible and affordable assay based on Real-time Reverse Transcriptase-Polymerase Chain Reaction technique (RT-PCR) for quantification of plasma HIV-1 RNA levels in patients with HIV infection. The real-time PCR technology measures accumulation of PCR product in real-time providing better quantification of the initial copy numbers with a dynamic range of detection. We designed and developed a two-tube method in which one tube is used for reverse transcription of HIV-RNA extracted from plasma/serum of HIV infected individual and the second tube is used for amplification of the transcribed cDNA. The use of Taqman probe contributes in measuring reporter dye emissions accurately with almost 100% specificity and sensitivity since it has already been reported that a single mismatch can lead to reduced amplification efficiencies resulting in decreased viral load measurements. Our assay is highly specific as all HIV-1 negative healthy donor samples failed to show any positive fluorescent signal. In addition the assay has a very high sensitivity and it can detect 30 g. eq./ml of HIV RNA. The standard curve showed linearity over eight orders of magnitude with high coefficient of correlation indicating its high sensitivity in determining viral loads over a broad range.
Uniqueness of this test resides in
- It quantitated almost all subtypes of HIV-1 including HIV-1 subtype O, particularly ● subtype C and subtype B;
- False positive results have been reduced to negligible levels;
- False negative results due to inhibition of amplification reaction or due to inappropriate extraction procedure have been avoided completely by inclusion of internal control which is added to the sample and is co-extracted with the virus;
- Quantitation standards are used with every run for quantitation. The dynamic range of the assay makes it particularly useful for quantifying full range of viral loads observed in treated and untreated patients with HIV infection.
Above all the reports are available within 24 hours of the receipt of the sample.
Most Common Reasons for Ordering Plasma Viral Load Tests
- History and symptoms consistent with acute HIV syndrome
- Indeterminate HIV antibody test in a patient at high risk for HIV infection
- Initial evaluation of newly diagnosed HIV infection
- Surveillance of patients who are not receiving antiretroviral drug therapy
- Before initiation or change of antiretroviral drug therapy
- Monthly after initiation of antiretroviral drug therapy and every 1 to 3 months until therapeutic goal is attained
LABORATORY Tests for HIV/AIDS
HIV diagnostic testing has come a long way since its inception in the early 1980s.
Current enzyme immunoassays (EIA) are sensitive enough to detect antibody as early as one to two weeks after infection. A variety of other assays are essential
- To confirm positive antibody screens (Western blot, polymerase chain reaction [PCR]),
- Provide an adjunct to antibody testing (p24 antigen, PCR), or
Provide additional information for the clinician treating HIV-positive patients (qualitative and quantitative PCR, and genotyping).
A brief review of the significance of these tests and their interpretation is discussed.
ELISA
 This assay detects the presence of antibodies to HIV-1 and HIV-2 in serum or plasma. The production of HIV specific antibodies after exposure to the virus takes about 612 weeks (seroconversion). The period between infection and seroconversion is called the window period. During this period ELISA for HIV antibodies is negative. The window period usually does not exceed 3-4 months.
This assay detects the presence of antibodies to HIV-1 and HIV-2 in serum or plasma. The production of HIV specific antibodies after exposure to the virus takes about 612 weeks (seroconversion). The period between infection and seroconversion is called the window period. During this period ELISA for HIV antibodies is negative. The window period usually does not exceed 3-4 months.
Detection of HIV-1 antibodies is the most common and efficient method of determining whether an individual has been infected with the virus.
Currently available enzyme immunoassays (EIAs) have analytical sensitivities and specificities that exceed 99%.
INTERPRETATION
Non Reactive: Implies that no HIV-1 or HIV-2 antibodies have been detected in the sample by this method. This means that either the patient has not been exposed to HIV-1 or HIV-2 infection or the sample has been tested during the window phase.
Provisionally Reactive/Borderline Reactive: Suggests the possibility of HIV-1 and/or HIV-2 infection. The reactive EIA result should be validated by the confirmatory Western Blot assay as false positive EIA results may occur under several conditions; for example
False Positive reactions: 1. High levels of IgM antibodies, 2. Anti HLA-ABC and DR antibodies, 3. Attack of Flu, 4. Hepatitis B vaccination, 5. Repeated freezing and thawing of the sample, 6. Multigravida women, 7. Presence of Rheumatoid factor, 8. Alcoholism, 9. Malignancy
False Negative Reactions: 1. Window Period, 2. Advanced AIDS disease – diminished antibody titre, 3. When the patient is immunosuppressed.
WESTERN BLOT (WB)
Western blot or Immunoblot is used to validate the results of repeatedly reactive EIA. It also identifies the antibodies directed against specific HIV-1 &/or HIV-2 antigens. It has a lower positive predictive value when performed alone. The Western blot is interpreted as positive, indeterminate or negative on the basis of the band-pattern (number & type of bands) on the assay strip. (See below “Interpretation)
The Western Blot analysis is currently the standard method for confirming HIV seropositivity. HIV proteins are separated according to their molecular weight by polyacrylamide gel electrophoresis and then transferred on to a nitrocellulose membrane. The antibodies present in the patient’s serum or plasma bind to these antigens. The specificity of this test is nearly 99%. The positive predictive value of Western blot assay in conjunction with EIA is 99%.
INTERPRETATION
The recommended WHO criteria for interpreting Western Blot results are as follows:
POSITIVE: Presence of two envelope bands (gp160/120 and gp 41) with / or without pol (p51/p66/p31) or gag bands (p55/p24/p17) OR the presence of at least one band from each gag, pol and envelope antigens.
A positive blot indicates infection with the virus but a diagnosis of AIDS can only be made clinically if the person meets the case definition of AIDS
( See given NACO guidelines, Oct 1999).
INDETERMINATE: Any band pattern which does not meet the criteria for positive is termed as Indeterminate. All such cases must repeat the test after 6 weeks. Indeterminate results can occur due to
- An incomplete antibody response in sera of infected persons or
- Nonspecific reactions in sera from uninfected persons.
NEGATIVE: No viral specific bands present is termed as Negative
INDICATED: Specific band for HIV-2 present is termed as HIV-2 indicated
NEGATIVE: Specific band for HIV-2 absent.
False Negative Results:
- Recent infection with HIV in the process of seroconversion
- End Stage HIV disease
- Perinatally exposed infants who are seroconverting i.e.losing maternal antibody
- Pregnant women
Note: All these causes lead to incomplete antibody response which makes the result Indeterminate or Negative.
False Positive Results: Contamination of viral antigen reference bands by histo-compatibility and other antigens during kit preparation. Uninfected infant born to HIV positive mother
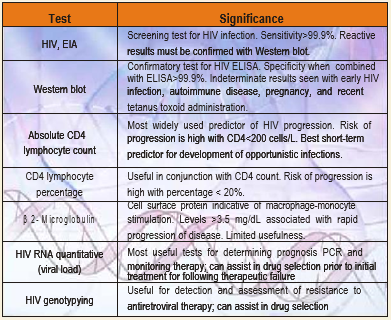
P 24 Antigen Detection: This test predicts the probability of disease progression. In seroconversion illness the test may be positive even before the . It takes 23 weeks after HIV infection for the antigens to first appear and remain detectable upto 5 months post infection. Thus antigen testing is strongly recommended for early detection of HIV infections in persons at risk. After seroconversion i.e. when HIV antibodies develop which takes about 6-8 wks usually (614 months in some individuals), the free HIV antigens in the blood disappear as they form immune complexes with the antibodies. The reappearance of the antigen is an unfavourable prognostic sign for the development of AIDS.
Uses: 1. Used for early detection of HIV cases before seroconversion, 2. Used as an aid in the prognosis and therapy of HIV positive individuals, 3. Used to detect HIV positivity in infants born to HIV infected mothers, 4. Used in the detection of HIV viral antigens on virus isolation in tissue cultures
HIV-1 DNA PCR: PCR is particularly useful in testing infants of HIV-positive mothers; these infants may carry maternal antibody to 15 months of age. It is also useful when testing patients who are agammaglobulinemic or in rare cases where patients appear to have symptoms of advanced HIV infection but do not demonstrate HIV-specific antibodies.
HIV Viral Load Monitoring: Aside from diagnostic HIV testing, LPL also offers quantitative PCR (RNA) testing (See HIV virus load by RT-PCR assay), which is used to help determine the initiation of drug therapy and monitor the effectiveness of therapy.
HIV Genotyping: HIV genotyping is a newer adjunct to patient management and is used to assist in tracking the development of drug resistance and guide the modification of antiretroviral drug selection .
Clinical Use: This test is used to detect HIV-1 mutations in the reverse transcriptase (RT) and protease (Pr) genes. Therefore, genotyping can be used to identify mutations associated with current or evolving resistance and to monitor transmission of drug-resistant HIV-1. Clinical evidence strongly supports the use of genotypic resistance testing to help guide therapy decisions in patients who have experienced virologic failure. Such guidance yields greater viral suppression than when therapy selection is based on standard of care, particularly when expert consultation is available
Other laboratory tests for monitoring HIV infection/ AIDS: 1. Routine Haematology and Biochemistry, 2. Total CD4 Count, 3. Total CD8 count, 4. CD 4/CD 8 Ratio, 5. Beta 2 Microglobulin, 6. Neopterin
These tests are recommended for the follow up of drug therapy and to rule out drug toxicity. Many HIV infected persons have cytopenias and the incidence increases with the use of concomitant drugs. Some patients develop rapidly progressing pancreatitis. Hence routine screening is essential.
Total CD4 Count
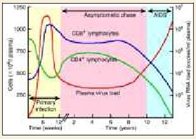 The most widely used prognostic marker has been the absolute CD4+ lymphocyte count. In general, as counts decrease, the risk of opportunistic infection increases. However, there is substantial diurnal variation (counts are generally lower in the morning), & counts may be depressed by the occurrence of an illness. The trend in counts is more important than any single value. The frequency of performance of counts depends on the patient’s health status. Patients early in the course of infection should have counts performed every 3 months. CD4 cell counts are the best immediate or short-term predictor of the risk of developing a new opportunistic infection. The percentage of CD4+ lymphocytes is a more reliable indicator of prognosis than the absolute counts because the percentage does not depend on calculating a manual differential.
The most widely used prognostic marker has been the absolute CD4+ lymphocyte count. In general, as counts decrease, the risk of opportunistic infection increases. However, there is substantial diurnal variation (counts are generally lower in the morning), & counts may be depressed by the occurrence of an illness. The trend in counts is more important than any single value. The frequency of performance of counts depends on the patient’s health status. Patients early in the course of infection should have counts performed every 3 months. CD4 cell counts are the best immediate or short-term predictor of the risk of developing a new opportunistic infection. The percentage of CD4+ lymphocytes is a more reliable indicator of prognosis than the absolute counts because the percentage does not depend on calculating a manual differential.
BETA 2 MICROGLOBULIN
β 2-Microglobulin, a marker for microphage and monocyte stimulation, is a cell-surface protein whose concentration increases at the time of HIV seroconversion and continues to rise with progression of disease. The test is not very useful; CD4 cell counts and viral load assays are stronger prognostic and therapeutic markers.
NEOPTERIN
Role in HIV: This is a marker of macrophage and T cell activation. This test also predicts probability of disease progression but the test has not been established as having additional value compared with total CD4 counts. High levels correlate with progression of the disease and the levels fall after therapy. Levels of neopterin can be measured in serum or urine. Elevated serum levels may indicate HIV infection in children with indeterminate status. Increased CSF neopterin is associated with neurologic disease in HIV infected individuals.
LABORATORY TESTS FOR HIV-1 INFECTION
DIAGNOSING HIV INFECTION IN CHILDREN
Diagnosis of HIV infection in children born to HIV-infected mothers is complicated by the presence of maternal anti-HIV IgG antibody, which crosses the placenta to the fetus. Virtually all these children are HIV-antibody positive at birth, although only 15%-30% are actually infected. In uninfected children, this antibody usually becomes undetectable by 9 months of age but occasionally remains detectable until 18 months of age. Therefore, standard anti-HIV IgG antibody tests should not be used to indicate reliably a child’s infection status before 18 months of age. Polymerase chain reaction (PCR) and virus culture are probably the most sensitive and specific assays for detecting HIV infection in children born to infected mothers. Isolation of HIV, though a gold standard, is highly infectious, labour intensive and time consuming procedure that requires special biological containment laboratory facility and highly skilled expertise and is conducted by a few laboratories in India. On the other hand, PCR is safe and provides results within 24 hours of receipt of sample. It is used as an alternative gold standard to confirm the diagnosis of HIV infection. PCR can identify approximately 30%-50% of infected infants at birth and nearly 100% of infected infants by 3-6 months of age.
The standard p24-antigen assay is less sensitive than either virus culture or PCR, especially when anti-HIV antibody levels are high, because it fails to detect immune-complexed p24 antigen. Though fourth generation ELISA kits can detect immune complexed p24 antigen, the overall sensitivity of this assay is about 50 to 60%.
Children born to mothers with HIV infection are defined as seroreverters (SRs) and are considered uninfected with HIV if they
- Become HIV-antibody negative after 6 months of age,
- Have no other laboratory evidence of HIV infection, and
- Have not met the AIDS surveillance case definition criteria. (See below, NACO guidelines for case definition of AIDS in India.)
Diagnosis of HIV infected children over 18months may be made by antibody test (ELISA and confirmatory tests)
Specific diagnosis in children less than 15 -18months can be made by virologic tests
- HIV DNA polymerase chain reaction (PCR)
- HIV RNA Assay
- Standard and immune complex dissociated p24 antigen
- Viral culture
Tests should be performed at: 48 hours of age, 14 days, 1-2 months, 3-6 months, Cord blood should be excluded
DIAGNOSIS OF HUMAN IMMUNODEFICIENCY VIRUS (HIV) INFECTION IN CHILDREN
- A child <18 months of age who is known to be HIV seropositive or born to an HIV-infected mother and:
has positive results on two separate determinations (excluding cord blood) from one or more of the following HIV detection tests:
HIV culture,
HIV polymerase chain reaction,
HIV antigen (p24), or
meets criteria for acquired immunodeficiency syndrome (AIDS) diagnosis based on the NACO guidelines, October 1999
- A child > 18 months of age born to an HIV-infected mother or any child infected by blood, blood products, or other known modes of transmission who:
- is HIV-antibody positive by repeatedly reactive enzyme immunoassay (EIA)
- and confirmatory test (e.g., Western blot or immunofluorescence assay {IFA}) or meets any of the criteria in (A)
A child who does not meet the criteria above and who:
- is HIV seropositive by EIA and confirmatory test (e.g., Western blot or IFA) and is <18 months of age at the time of test, or
- has unknown antibody status, but was born to a mother known to be infected with HIV
A child who is born to an HIV-infected mother and who:
- Has been documented as HIV-antibody negative (i.e., two or more negative EIA tests performed at 6-18 months of age or one negative EIA test after 18 months of age) and
- Has had no other laboratory evidence of infection (has not had two positive viral detection tests, if performed) and
- Has not had an AIDS-defining condition.
NACO GUIDELINES -CASE DEFINITION FOR AIDS IN INDIA (October 1999)
- The positive tests for HIV infection by ERS test (ELISA/RAPID/SIMPLE) in children above 18 months, or
- confirmed maternal HIV infection for children less than 18 months. and
- Presence of at least two major and two minor signs in the absence of known causes of immuno-suppression.
MAJOR SIGNS:
- Loss of weight or failure to thrive which is not known to be due to medical causes other than HIV infection.
- Chronic diarrhea (intermittent or continuous) > 1 month duration.
- Prolonged fever (intermittent or continuous) > 1 month duration.
MINOR SIGNS:
- Repeat common infections (e.g. Pneunonitis, ottitis, pharyngitis etc.)
- Generalised lymphadenopathy
- Oropharyngeal candidiasis
- Persistent cough for more than 1month
- Disseminated maculo – papular dermatosis
- Two positive tests for HIV infection by ERS test (ELISA/RAPID/SIMPLE) and
- Any one of the following criteria:-
- Significant weight loss (> 10% of body weight) within last one month/Cachexia (not known to be due to a condition other than HIV infection) and Chronic diarrhea (intermittent or continuous) > 1 month duration or prolonged fever (intermittent or continuous) > 1 month duration
- Tuberculosis: Extensive pulmonary, disseminated, miliary, extra-pulmonary tuberculosis.
- Neurological impairment preventing independent daily activities, not known to be due to the conditions unrelated to HIV infection (e.g. trauma)
- Candidasis of the oesophagus (diagnosable by oral candidiasis with odynophagia)
- Clinically diagnosed life -threatening or recurrent episodes of pneumonia, with or without etiological confirmation
- Kaposi Sarcoma
- Other conditions:-
● Cryptococcal meningitis ● Neuro Toxoplasmasis
● CMV retinitis ● Pencillium marneffei
● Recurrent Herpes Zoster or ● Disseminated molluscum
multi-dermatomal herpes infection