The Importance of HLA Antibodies in Transplantation


Historically, anti-Human Leukocyte Antigen (HLA) antibodies were defined as preformed circulating antibodies present in the recipient’s immune system which were the result of a sensitization event pre-transplant (blood transfusion, previous transplant or pregnancy). In more recent years, the concept of monitoring the post-transplant development of clinically relevant antibodies directed against donor specific HLA class I and class II mismatches has been a significant area of interest within the transplant community.
Whether detected pre or post-transplantation, the presence of antibodies directed against antigens expressed on donor cells, when not treated clinically, result in an immune attack on the transplanted organ and increases the risk of graft loss and / or rejection.

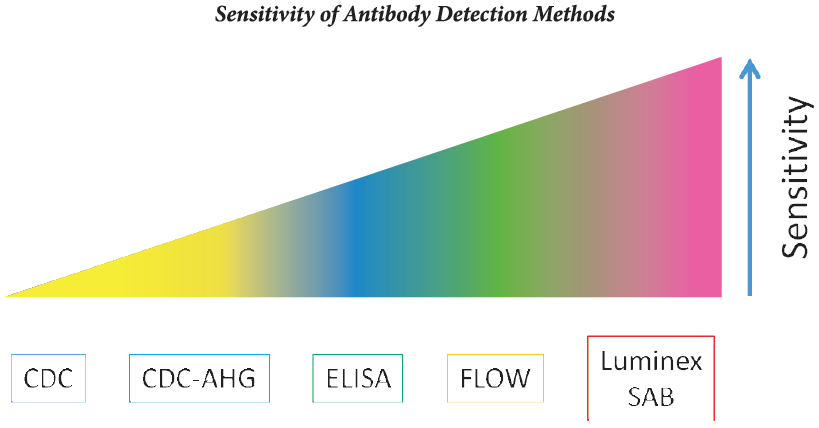
The presence of preformed anti-HLA Donor Specific Antibodies (DSA) is a major risk factor for early renal allograft rejection and graft loss [1,2]. Different techniques are currently available to detect DSA in sera of patients awaiting a graft.
• The standard Complement-dependent Cytotoxicity Cross-match (CDCXM) and modifications of this technique such as the Anti-human Globulin-augmented Assay (AHG-CDCXM) have been most widely used to detect DSA before transplantation 13-51. The widespread utilization of CDCXM against donor T lymphocytes has resulted in almost complete elimination of hyperacute rejectioni6-81.
• The Flow Cytometric Cross-match (FCXM) is more sensitive than CDCXM [‘-8); nevertheless, this technique is not specific because it can detect antibodies directed against cell surface antigens irrelevant in the outcome of the graft. The issue of FCXM being a more sensitive but less specific technique compared with CDCXM, leads to false-positive results, preventing the opportunity of a transplant in patients without relevant DSA.
• The Single Antigen Bead Flow Cytometry (SAFC) allows the determination of the presence of DSA comparing the HLA typing of the donor with the recipient of anti-HLA antibodies against recombinant HLA molecules in the serum of each patient.

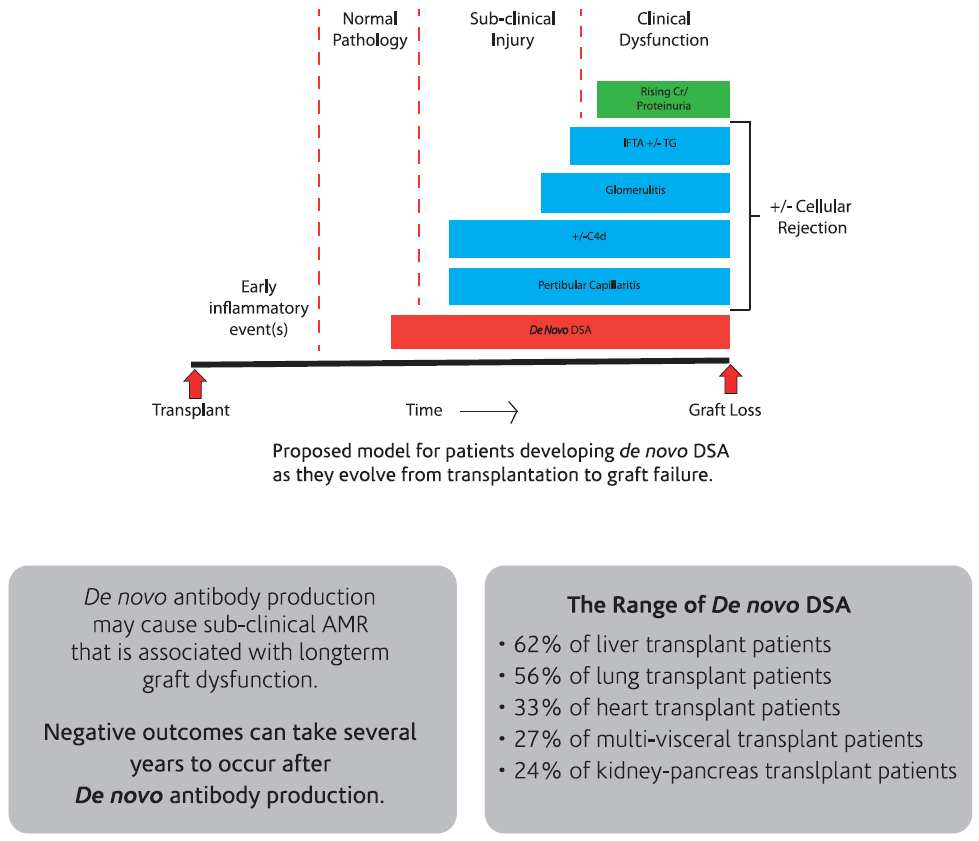
Antibody mediated rejection can present as an early acute process, often resulting from an anamnestic response, or as a late and chronic process due to de novo antibody production. In the acute phase, it is often pre-formed antibodies that cause early rejection. De novo DSA can also develop in the early post-transplant period, resulting in acute rejection. Patients with preformed DSA are at significantly greater risk of having an acute AMR and have significantly lower graft survival9.

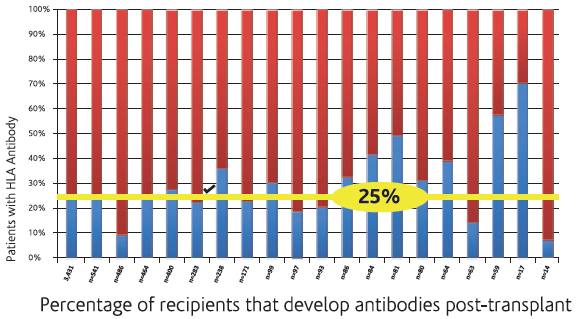
The development of de novo donor-specific HLA antibodies (dnDSA) post-transplantation has been associated with higher graft failure rates. The range of dnDSA is 24% to 62% with the highest presence in post liver transplant recipients.
Several prospective and retrospective studies have demonstrated that de novo DSA development in renal transplant patients, predominately directed at Class II donor HLA mismatches, is associated with poor outcomes and is often detectable well before graft failure [10,11].

The routine monitoring of DSA may provide early identification of those patients at risk for rejection due to insufficient immunosuppression as a result of poor compliance with immunosuppressive therapy. DSA monitoring may be useful in the adjustment of immunosuppressive therapy post-transplant. The close surveillance of humoural immunity is mandatory for the precise adjustment of anti-humoral therapy and optimal Outcomes13 .
Studies have shown that when DSA is identified early through routine monitoring , the initiation of anti-humoural therapy may begin sooner, allowing for DSA to clear and improving overall survival rates. Survival is known to be significantly worse in recipients who have persistent DSA than those who clear the DSA12.

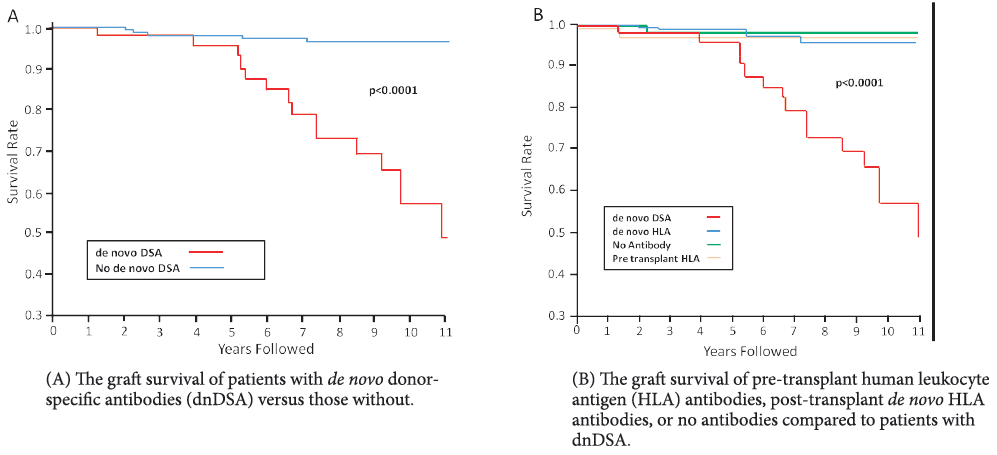




Longitudinal monitoring of DSA is more valuable than single-point testing, particularly in the post-transplant setting, and is crucial in optimizing patient outcomes9.
Kimball et al. performed quarterly monitoring of patients for 3 years post-transplant and reported that 65% of patients eliminated antibody within the first year of transplant due to early detection and subsequent treatment. In those patients that exhibited pre-transplant DSA against Class I and II HLA, antibody levels declined by 90% at 6 months and were undetectable at 1 year with effective treatment16.

British Transplantation Society and British Society of Histocompatibility Guidelines for DSA17
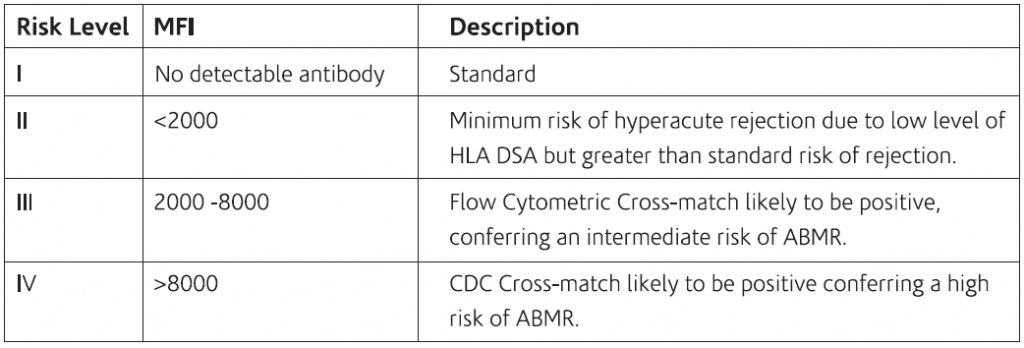
Multi-visceral transplantation: The table below shows the level of risk of developing ABMR along with MEI in Multi-visceral transplantation.
Frequency of testing for DSA / SAB :
• At three monthly intervals; also antibody screening and specificity testing should be done on two separate samples at different time points.
• HLA Class II antibodies pose a significantly higher risk of rejection for both kidney and liver transplant recipients.
Post-transplant monitoring in recipients with preformed DSA
Luminex is able to identify preformed DSA in more recipients. Transplants in patients with DSA identified by CDC cross-match are contraindications to transplants. A positive flow cross-match and DSA detected by SAB indicate higher risk of acute and chronic ABMR and patients may require desensitization. Post-transplant anamnestic response can lead to rise in DSA titers. Unsensitized recipients may also show presence of DSA associated with graft dysfunction.
University of Michigan recommendations
Frequency of post-transplant DSA test:
1. High risk — includes those with PRA >20%, re-transplants, history of acute cellular rejections: at 3, 6 and 12 months post-transplant.
2. De novo DSA may be transient and if low titer requires close monitoring; high titer DSA or DSA in setting of stable renal function requires allograft biopsy.

While implementation of routine post-transplant monitoting becomes increasingly recognized as a standard-of-care practice, the frequency of testing is highly variable. The frequency of post-transplant monitoring will be to a large extent, patient-specific. Choosing a monitoring frequency based on a patient’s individual risk of developing AMR post-transplant will be the most efficient and clinically relevant strategy.
More recently, data has been reported, indicating that the identification and subsequent treatment of DSA in the post-transplant setting, for all solid organ types, may be an important consideration in the long-term treatment of the transplant patient. The routine monitoring of DSA in the post-transplant setting offers promise in long-term graft and patient survival.

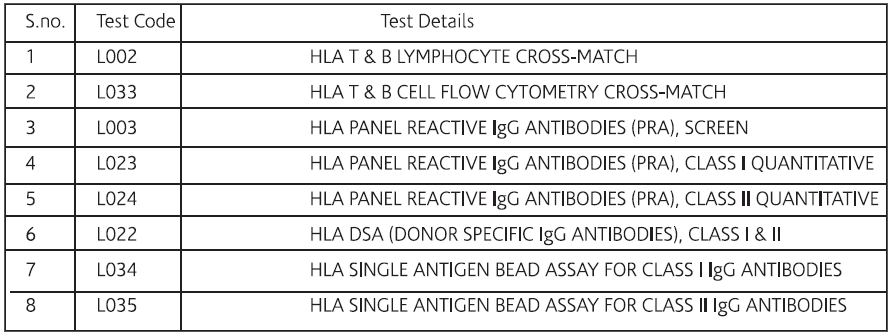
For Pre & Post Solid Organ Transplant

1. Gebel HM, Bray RA, Nickerson – Pre-transplant assessment of donor-reactive, HLA-specific antibodies in renal transplantation.
2. Class FHJ-Clinical relevance of circulating donor-specific HLA antibodies. Curr Opin Organ Transplant.
3. Johnson AR, Rossen RD, Butler WT-Detection of alloantibodies using a sensitive antiglobulin microcytotoxicity test: identification of low levels of preformed antibodies in accelerated allograft rejection.
4. Terasaki PI, McClelland JD-Microdroplet assay of human serum cytotoxins.
5. Kissmeyer-Nielsen F, Olsen S, Petersen VP, et al-Hyperacute rejection of kidney allografts, associated with pre-existing humoral antibodies against donor cells.
6. Iwaki Y, Lau M, Cook DJ, et al-Crossmatching with B and T cells and flow cytometry.
7. Scornik JC-Detection of alloantibodies by flow cytometry: relevance to clinical transplantation. Cytometry.
8. Patel AM,Pancoska C, Mulgaonkar S, et al-Renal transplantation in patients with pre-transplant donor-specific antibodies and negative flow cytometry crossmatches.
9. Lefaucheur, et al. Clinical relevance of preformed HLA donor-specific antibodies in kidney transplantation. American journal of transplantation.
10. Wiebe C, et al Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. American Journal of Transplantation.
11. Fotheringham J, et al Natural history of protienuria in renal transplant recipients developing de novo human leukocyte antigen antibodies.
12. Hachem RR, et al. Anti-human leukocyte antigen antibodies and preemptive antibody-directed therapy after lung transplantation.
13. Tsai HL, et al. Association between donor-specific antibodies and acute rejection and resolution in small bowel and multivisceral transplantation.
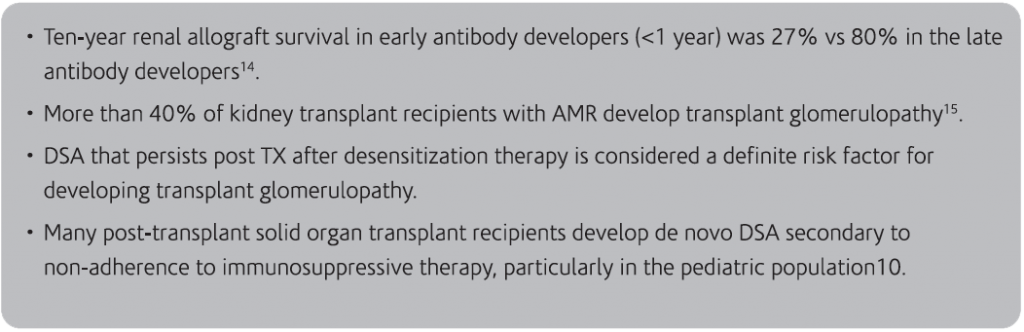
14. Lee PC, et al. HLA-specific antibodies developed in the first year post-transplant are predictive of chronic rejection and renal graft loss.
15. Gloor JM, et al. Histologic findings one year after positive cross match or ABO blood group incompatible living donor kidney transplantation.
16. Terasaki, et al. Humoral theory of transplantation.
17. www.bts,org.uk & wwww.bshi.org.uk
18. Hachem RR, et al. Anti-human leukocyte antigen antibodies and preemptive antibody-directed therapy after lung transplantation.
19. Smith JD, et al. De novo donor HLA-specific antibodies after heart transplantation are an independent predictor of poor patient survival.
20. Tsai HL, et al. Association between donor-specific antibodies and acute rejection and resolution in small bowel and multivisceral transplantation.
21. O’Leary, et al. High mean fluorescence intensity donor-specific anti-HLA antibodies associated with chronic rejection post liver transplant.
22. Loupy A, et al. Outcome of subclinical antibody-mediated rejection in kidney transplant recipients with preformed donor-specific antibodies. American Journal of Transplantation.













