TB Diagnosis – An Overview
TB LAB DIAGNOSIS IN 2014
India is a high TB burden nation. Every 5th case of tuberculosis globally is an Indian. Care of patients with tuberculosis (TB) starts with a quality assured diagnosis. Laboratory diagnosis of tuberculosis has 3 options ie: you see the bug (microscopy), you grow the bug (culture) and you multiply the bug (PCR). These are the only WHO endorsed methods to detect tuberculosis in the patient suffering from active tuberculosis infection. For this to become effective and successful one has to send the site specific sample. There is no room for blood based test for diagnosing a case of active tuberculosis. The consequences of relying on blood tests are terrible — patients who are actually not suffering from active TB are put on long-term medication, while many people who are actually suffering from active TB never end up getting the treatment at the right time. TB patients who are not undergoing treatment can spread the disease to a large number of people.
The algorithm for TB lab diagnosis begins with a good site specific sample. The first test in this category is microscopy which is done by two methodologies ie: Auramine screen and confirmed by ZN Stain.
Specificity of microscopy is 100%.
However, smear microscopy has some inadequacies:
• Sensitivity of microscopy is poor (20-30%) and for it to be positive there has to be minimum bacterial load of 10000 bacilli/ml.
• Is prone to false-negative ‘smear-negative TB’ results
• It cannot test for drug resistance, which is important because patients with drug-resistant TB have high mortality.
Also the technique is not rewarding for extra pulmonary samples.
The next test in this algorithm is TB culture which is also regarded as the gold standard test for tuberculosis. Liquid based automated culture method is done at Dr Lal path lab in a state of the art BSL-3 laboratory.
Sensitivity of culture is minimum 100 bacilli/ml. Both sensitivity and specificity of the test is >98%.
Culture growth is important to not only know the type of Mycobacterium but also to work up further for drug sensitivity. At present we are testing 5 drugs from first line and 10 drugs from second line. All molecular based test reporting resistance should be confirmed by culture based DST. As per WHO incidence of primary drug resistance in India is around 3 % and secondary drug resistance is 17 %.
The last test is this series is MOLECULAR BASED test. We have in house PCR TEST which is validated and CAP approved test with a sensitivity of > 98% in a smear positive case and around 70% in a smear negative case of tuberculosis. Again site specific sample is very important to get a good result from a TB PCR Test. Blood as a sample has no role in TB PCR, infact it is a natural inhibitor of this test. TB PCR not only identifies the type of Mycobacterium but also provides information about the drug resistance.
Advantage with PCR is speed as it provides complete information same day. This is important if the physician wants to rule out MDR STATUS. Genotypic methods have considerable advantages for scaling-up programmatic management of drug-resistant and HIV-associated TB, in particular with regard to speed, standardised testing, potential for high throughput. GeneXpert MTB/RIF detects both TB and rifampicin resistance in a single test in 2 hrs. This test is done on the direct sample. It can be performed on pulmonary and extra pulmonary specimen, smear positive as well as smear negative specimens. Rifampicin resistance is a good and reliable proxy for MDR-TB in high burden settings. Xpert MTB/RIF can be used as a stand-alone diagnostic test in individuals at risk of MDR-TB.
Line probe assay or Hain assay is useful in a smear positive case or culture positive case of tuberculosis. It cannot be applied on a direct specimen. However the advantage is it speciates’ Mycobacterium and covers drug resistance detection for both first and second line drugs. So if Genexpert detects rifampicin resistance then detailed work up can be performed by Line probe assay in a short span of 1-2 days.
Role of Interferon gamma release assay (IGRA) in detecting an active case of tuberculosis is nil. It does not differentiate between the active and chronic disease. So this test should not be used solely to diagnose and treat tuberculosis infection. However there are certain situations like where site specific sample is not defined or difficult to extract or lethal to extract or clinical, radiological or other supplemental markers like ESR/ ADA levels raise suspicion for tuberculosis, IGRA can be used to rule out exposure to a tuberculosis infection.
Dr. Shalabh Malik, MD
HOD Microbiology and Clinical Pathology
National Reference Laboratory
Dr Lal PathLabs Pvt Ltd
Role of PCR in the Diagnosis of Tuberculosis
India ranks first worldwide in reporting the highest number of new tuberculosis cases per year.
Crucial to containing the spread of infection is early, rapid and accurate diagnosis of pulmonary TB.
Xpert gives results within 2 hours of loading the sample on the analyser.
Generally for TB PCR
(i) Results are available in 24 —48 hours
(ii) In 80%-90./0 of suspected cases, TB is detected weeks earlier by PCR than confirmed by culture (ii) PPV >95% with AFB smear positive specimens
(iii) Rapid confirmation of presence of MTB in up to 80% of smear negative, culture positive specimens
CDC guidelines for the Use of NAA Tests in the Diagnosis of TB
(Published in MMWR January 16, 2009 / 58(01):
- Culture must be done in all cases
- NAA should be performed on at least one respiratory sample; preferably the first
- NAA results are to be interpreted in correlation with AFB smears
Interpretation of results
- NAA+ AFB+ Presume TB + start treatment
- NAA + AFB – use clinical judgment whether to start ATT while waiting for culture results
- NAA – AFB + Test for inhibitors ( 3%-7%) if inhibitors not detected repeat NAA & AFB and if both are negative on repeat sample presume NTM infection
- NAA- AFB- Use clinical judgment while waiting for culture results
Caution
- Culture remains gold standard and is required for AST
- Singly negative NAA should not be used to exclude TB infection especially if clinical suspicion is moderate to high
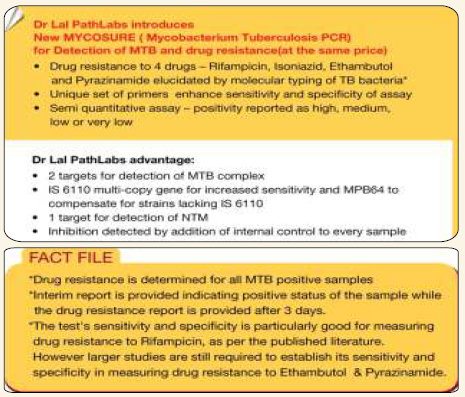
Mycosure TB PCR
This assay for the diagnosis of TB was developed in the research laboratory at Dr. Lal PathLabs. In 2004 the first version was launched using the 16s gene as the target and liquid hybridization for detection; 2 probes were used; one probe identified the TB complex & the second probe identified the Mycosure genus.
In 2009 a new version of Mycosure TB PCR was launched using real time PCR technology based on the Taqman principle
- Detection of MTB complex is based on two targets — IS 6110 and MPB 64
- IS 6110 is multicopy gene — targeted by most PCR’s due to its enhanced sensitivity, BUT has been found lacking in India strains
- MPB 64 — compensates for the strains lacking IS 6110
- Detection of NTM is based on 16s gene
- Detection of inhibitors of PCR by addition of Internal positive control (IPC)
- Reflex testing is performed for drug sensitivity of Rif, INH, Ethambutal and Pyrzinamide
Xpert® MTB/RIF
Is a WHO endorsed system for rapid detection of MTB in pulmonary samples
In addition to the speed of analysis, there is simultaneous detection of both MTB and rifampicin resistance which is a surrogate marker for MDR strains. Inhibition is also detected.
There is no requirement for additional instruments other than the GeneXpert® System.
Another advantage is ease of operation.
- Overall Sensitivity Culture Positive: 92.1% a
- Sensitivity AFB+/Culture+: 97.9%
- Sensitivity AFB-/Culture+: 73.1% (Single Sputum)
- 90.0%(3 Sputum Samples)
- Specificity: 99.0%
XPERT MTB/RIF ASSAY COMPARED TO DRUG SUSCEPTIBILITY TESTING FOR RIFAMPICIN
- Sensitivity: 97.2%
- Specificity: 98.3%
A limitation of this system is its inability to detect NTM which can be pathogenic.
Inhibition
- A limitation of nucleic acid testing is inhibition
- It is important to detect inhibition in order to rule out false negative results
- Inhibitors present in the specimen inhibit the amplification step in the PCR reaction
- It is detected in upto 7% of samples
- Inhibition is detected by adding an Internal control to every sample
- Internal control is a known target sequence
- Failure of internal control to amplify demonstrates presence of inhibitor
- Detection of inhibition invalidates the test
The diagnosis of tuberculous infection can be challenging particularly in non-pulmonary cases. Understanding the clinical utility as well as the limitations of the laboratory tests is important in interpretation of laboratory results.
I would recommend reading the following comprehensive review Clin Microbiol Rev. 2011 April; 24(2): 314-350.
Linda M. Parsons,’ Akos Somoskovi,2Cristina Gutierrez,’ Evan Lee,’ C. N. Paramasivan,2 Alashle Abimiku,’ Steven Spector,’ Giorgio Roscigno,2 and John Nkengasoncit
Dr. Anjali K. Kale
MBBS, DCP, MD,FCAP
Diplomate American Board of Pathology
Chief Consultant Pathologist
National reference laboratory
Dr. Lal PathLabs, New Delhi
As we mark 24th March as ‘World TB Day‘ on our calendars — we come to realize that even forty years after the first time anti-mycobacterial treatment became widely available, the world still has to cope with rising levels of TB incidence. In 2014, the slogan for World Tuberculosis Day is “Reach the 3 million“.
TB is curable, but current efforts to find, treat and cure everyone who gets ill with the disease are not sufficient. Of the 9 million people a year who get sick with TB, a third of them are “missed” by health systems.
India is already home to the highest number of TB sufferers globally with two million cases every year. In this number it is estimated that about 40% of the Indian population is infected with the TB and a vast majority have latent rather than active TB. It is not only the TB incidence that is worrying, but the fact that we are now battling an increasing number of drug-resistant strains of the dreaded infection. This situation is further worsened primarily because of the association between tuberculosis and HIV infection. With 1,000 Indians dying every day of TB, and with the highest number of TB patients in the world, India is undoubtedly the crucial battleground for TB control.
Improving diagnostic testing will go a long way in the combat against tuberculosis. Earlier and faster diagnosis of all forms of TB is vital It improves the chances of people getting the right treatment and being cured, and it helps stop spread of drug-resistant disease. Tuberculosis (TB) care and control does not end with an accurate and confirmed diagnosis, but that is a good start point. “Without diagnostics, medicine is blind.
In this issue Dr Anjali Kale and Dr Shalabh Malik share the details and guidelines to the utility of the WHO approved diagnostic tests in use. With the advent of new and faster technology to test for TB, this World Tuberculosis Day we can definitely hope that the next year we will achieve our goal of ‘Reach the 3 million’.