The Significance of QuantiFERON – TB Gold Test
WILL IT REVOLUTIONIZE THE DIAGNOSIS OF TB INFECTION ?
Tuberculosis (T B) Is a highly contagious respiratory disease caused by bacteria of the Mycobacterium tuberculosis complex.
Latent TB infection , which is a non-communicable asymptomatic condition results when a person becomes exposed to Mtb and their body controls, but does not eradicate the infection.
Immunosuppression, can result in re- activation of the Latent tuberculosis and development of tuberculosis disease. Diagnosis of Latent TB infection (LTBI) is an important step in preventing reactivation of tuberculosis, particularly in the risk populations and treatment of Latent TB can markedly reduce the risk of progression to active disease.
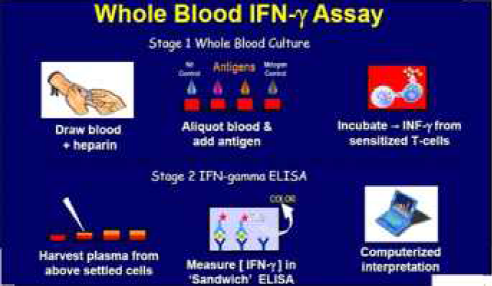
The Quantiferon TB Gold assay is an aid in –vitro in the diagnosis of Mtb infection. QFT-G is a type of INF-ã release assay conducted on sensitized white cells after whole blood is incubated with antigen. The FDA approved QFT-G is approved as an aid for diagnosing both active TB disease and LTBI. Previous studies have demonstrated a sensitivity of 80% for the QFT-G in persons with untreated culture-confirmed TB. The predictive value depends on the prevalence of TB in the community.
The test uses three recombinant peptides from Mtb ( ESAT-6,CFP-10 and TB7.7 ) to stimulate T cell interferon gamma production in individuals with Mtb infection. These peptide antigens do not usually stimulate lymphocytes from uninfected, BCG vaccinated persons without disease or risk for latent TB.
Previously,Tuberculin skin test ( TST / Mantoux reaction ) was the only method for assessing TB infection. Though widely used it has its own limitations like
- Poor inter-reader reliability – 9 mm (negative) vs. 10mm (positive)?
- False-positives/specificity
- NTM infection
- b. Prior BCG
- Cost/time of patient visits –
- Unread tests
- Sensitivity?
- Reaction wanes over time
- Lack of gold standard
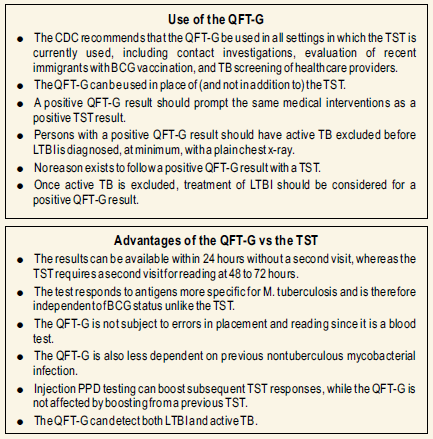
These limitations have been addressed in Quantiferon TB Gold assay.
- The stimulation of lymphocytes with Mtb antigens occurs in the phlebotomy tube, rather than in the patient’s arm. As a result there is no booster effect from repeated testing as in the case of tuberculin skin test.
- Results of Quantiferon TB Gold are available following a single patient visit without the need for a second visit to evaluate the skin test.
- The in vitro assay is not associated with adverse hypersensitivity reactions.
- The recombinant antigens chosen as stimulants in the new assay are not present in the BCG vaccine. Therefore, the assay is more specific and prior BCG vaccination will not cause false positives in this test, as it does in the tuberculin skin test.
- Inaddition the interpretation of the lab test is more objective.
This test is more sensitive and specific method for the assessment of tuberculosis infection both latent and in active disease.
Principle – If the patient is infected with M tuberculosis , the white blood cells will release Interferon gamma in response to contact with the TB antigens. The Quantiferon TB gamma results are based on the amount of interferon gamma that is released in response to the antigens.
Each quantiferon TB Gamma result and it’s interpretation should be considered in conjunction with other epidemiological, historical, physical, and diagnostic findings.
The advantages of the test are:
- Requires a single patient visit to draw a blood sample.
- Result can be available within 24 hours.
- Does not boost responses measured by subsequent tests , which can happen with tuberculin skin test.
- Is not subject to reader bias that can occur with tuberculin skin test.
- Is not affected by prior BCG.
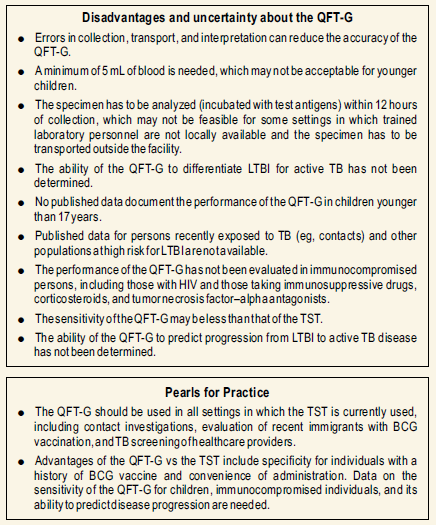
The disadvantages and limitations :
- Blood samples must be incubated with test antigens within 12 hrs after collection while white cells are still viable.
- There are limited data on the use of Quantiferon –gamma in children younger than 17 yrs of age, among persons recently exposed to M. tuberculosis, patients on immunosuppressive drugs, selected hematological disorders, specific malignancies, diabetes, silicosis, and chronic renal failure.
- Limited data is available on the use of Quantiferon –gamma TB test to determine who is at risk for developing TB disease
Like the TST, QFT-G cannot distinguish infection associated with TB disease from LTBI. For definitive diagnosis of LTBI, TB disease must be ruled out by medical evaluation, which should include ascertaining history of suggestive symptoms and signs, chest x-ray, and examination of sputum or other clinical samples for M. tuberculosis when indicated. As for other diagnostic tests, the prevalence of M. tuberculosis infection in the population being tested affects the predictive value of QFT-G results.
Conclusion
Specificity of Quantiferon TB-Gamma is more than Tuberculin skin test.
Sensitivity of Quantiferon TB- Gamma for TB disease is equal to Tuberculin skin test.
CDC GUIDELINES ON USE OF
QUANTIFERON TB GOLD TEST