Rapid Diagnostic Tests for Malaria
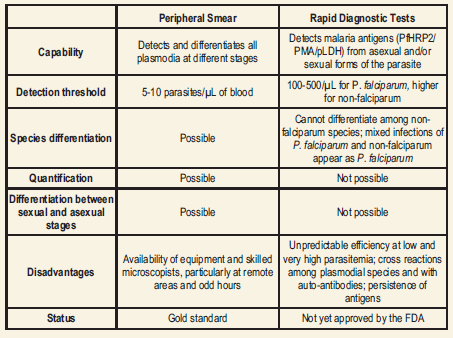
Although the peripheral blood smear examination that provides the most comprehensive information on a single test format has been the “gold standard” for the diagnosis of malaria, the immunochromatographic tests for the detection of malaria antigens, developed in the past decade, have opened a new and exciting avenue in malaria diagnosis.
Immunochromatographic Tests for Malaria
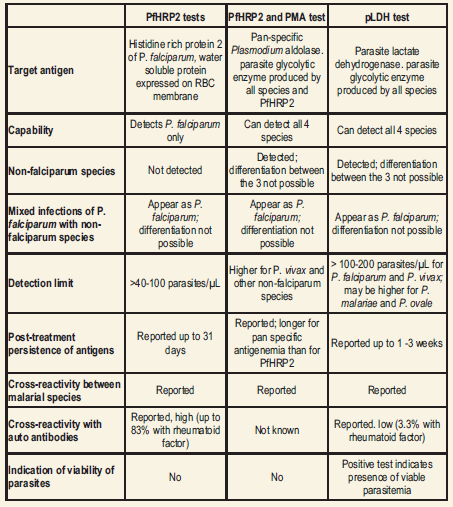
Antigens Immunochromatographic tests are based on the capture of the parasite antigens from the peripheral blood using either monoclonal or polyclonal antibodies against the parasite antigen targets. Currently, immunochromatographic tests can target the histidine-rich protein 2 of P. falciparum, a pan-malarial Plasmodium aldolase, and the parasite specific lactate dehydrogenase. These RDTs do not require a laboratory, electricity, or any special equipment.
Histidine-rich protein 2 of P. falciparum (PfHRP2) is a water soluble protein that is produced by the asexual stages and gametocytes of P. falciparum, expressed on the red cell membrane surface, and shown to remain in the blood for at least 28 days after the initiation of antimalarial therapy. Several RDTs targeting PfHRP2 have been developed.
Plasmodium aldolase is an enzyme of the parasite glycolytic pathway expressed by the blood stages of P. falciparum as well as the non-fa1ciparum malaria parasites. Monoclonal antibodies against Plasmodium aldolase are pan-specific in their reaction and have been used in a combined ‘P.f/P.v’ immunochromatographic test that targets the pan malarial antigen (PMA) along with PfHRP2.
Parasite lactate dehydrogenase (pLDH) is a soluble glycolytic enzyme produced by the asexual and sexual stages of the live parasites and it is present in and released from the parasite infectederythrocytes. It has been found in all 4 human malaria species, and different isomers of pLDH for each of the 4 species exist. With pLDH as the target, a quantitative immunocapture assay, a qualitative immunochromatographic dipstick assay using monoclonal antibodies, an immunodot assay, and a dipstick assay using polyclonal antibodies have been developed.
Principle
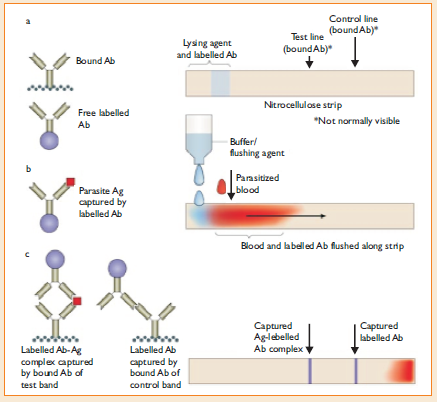
The RDTs have been developed in different test formats like the dipstick, strip, card, pad, well, or cassette; and the latter has provided a more satisfactory device for safety and manipulation.
The test procedure varies between the test kits.
In general, the blood specimen (2 to 50µL) is either a finger-prick blood specimen, anticoagulated blood, or plasma, and it is mixed with a buffer solution that contains a hemolyzing compound and a specific antibody that is labeled with a visually detectable marker such as colloidal gold.
In some kits, labeled antibody is pre-deposited during manufacture and only a lysing/washing buffer is added. If the target antigen is present in the blood, a labeled antigen/antibody complex is formed and it migrates up the test strip to be captured by the pre-deposited capture antibodies specific against the antigens and against the labeled antibody (as a procedural control).
Awashing buffer is then added to remove the hemoglobin and permit visualization of any colored lines formed by the immobilized antigen-antibody complex.
Problems with RDTs
- Cross-reactions with autoantibodies:
Studies have reported cross reactivity of the various RDTs with autoantibodies such as rheumatoid factor, resulting in false positive tests for malaria.False positive reactions are higher with the PfHRP2 tests using IgG capture antibody (16.5% to 83% ) compared to the PfHRP2 tests using IgM antibodies (6.6%) and the pLDH test (3.3%). Cross reactivity of the PMA antibody with rheumatoid factor does not appear to occur.
- Sensitivity:
- Depending upon parasite density: RDTs for the diagnosis of falciparum malaria generally achieve a sensitivity of >90% at densities above 100 parasites per µL blood and the sensitivity decreases markedly below that level of parasite density.Many studies have achieved >95% sensitivity at parasitemia of ~500 parasites/µL, but this high parasitemia is seen in only a minority of patients.
- Depending upon Kit used: For the diagnosis of vivax malaria, the PfHRP2/PMA test has a lower sensitivity compared to that for P. falciparum malaria; however, the pLDH test has an equal or better sensitivity for P. vivax malaria compared to P. falciparum malaria. For the diagnosis of P. malariae and P. ovale infections, the sensitivity is lower than that of
- falciparum malaria at all levels of parasitemia on both the PfHRP2/PMAand the pLDH tests.
- Specificity:
The specificity appears to be better with the pLDH test than the PfHRP2/PMA test for both P.falciparum and non-falciparum malaria.
- False Positivity
Potential causes for PfHRP2 positivity, other than gametocytemia, include persistent viable asexual-stage parasitemia below the detection limit of microscopy (possibly due to drug resistance), persistence of antigens due to sequestration and incomplete treatment, delayed clearance of circulating antigen (free or in antigen-antibody complexes) and cross reaction with non-falciparum malaria or rheumatoid factor. Proportion of persistent positivity has been linked to the sensitivity of the test, type of test, degree of parasitemia and possibly the type of capture antibody.
- False negativity
On the other hand, false negative tests have been observed even in severe malaria with parasitemias >40000 parasites/µl. This has been attributed to possible genetic heterogeneity of PfHRP2 expression, deletion of HRP-2 gene, presence of blocking antibodies for PfHRP2 antigen or immune-complex formation, prozone phenomenon at high antigenemia or to unknown causes.
- Cross reactions between Plasmodia species and problems in identifying non- falciparum species
Cross reaction of PfHRP2 with non-falciparum malaria could give false positive results for P. falciparum and mixed infections containing asexual stages of P. falciparum could be interpreted as negative in about one third of the patients.
Comparison of Rapid Diagnostic Tests for Malaria Antigens
Comparison of Peripheral Blood Smear Examination and RDTs for Malaria
Principle of RDT